Areas of Study
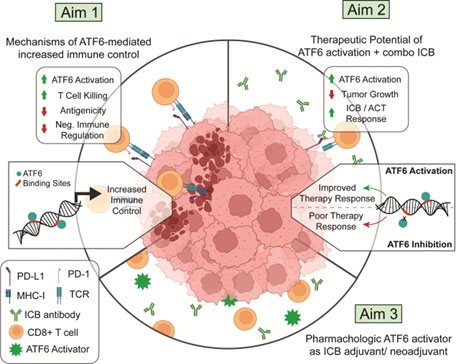
Activation of ATF6 in Melanoma to Increase Efficacy of Immunotherapy
Advancements in immune checkpoint blockade (ICB) and tumor-infiltrating lymphocyte (TIL) therapy have reshaped metastatic melanoma treatment, yet resistance remains a major challenge. We identified a novel role for Activating Transcription Factor 6 (ATF6) in sensitizing melanoma cells to T cell killing (TCK). ATF6, a key regulator of stress responses, enhances immune recognition and sensitizes tumor cells to TCK. Our data show that ATF6 activation, combined with ICB, cures a subset of non-responsive melanoma and induces immunological memory. We hypothesize that ATF6 activation promotes a transcriptional program that enhances TCK and ICB efficacy. To test this, we will: (1) define how ATF6 regulates immune control at the transcript, protein, and epigenetic levels, (2) evaluate its ability to enhance ICB and adoptive cell therapy (ACT) in vivo, and (3) assess pharmacologic ATF6 activation in a preclinical metastatic melanoma model.
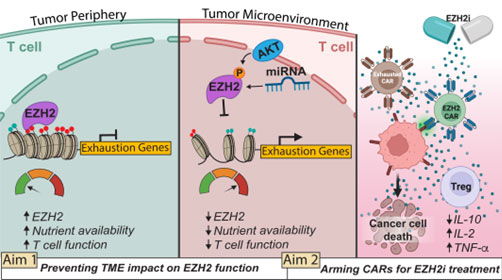
Leveraging cancer-evolved resistance mechanisms to enhance EZH2 activity in adoptive T cells
Metastatic melanoma treatment has been transformed by immune checkpoint blockade (ICB), yet resistance—often due to MHC-I antigen loss—limits its effectiveness to about half of patients. Adoptive cell therapy (ACT), such as CAR T cells, offers a promising alternative, but the tumor microenvironment (TME) imposes metabolic constraints that lead to T cell dysfunction and exhaustion. Our research focuses on enhancing T cell resilience in the TME by preserving EZH2, a histone methyltransferase whose loss drives T cell exhaustion. Our preliminary data suggest that protecting EZH2 activity sustains T cell function, supporting our hypothesis that EZH2 preservation will improve ACT efficacy. We will test this through two aims: (1) evaluating EZH2’s role in maintaining T cell function in the TME and (2) engineering EZH2i-resistant CAR T cells for combination therapy in metastatic melanoma.
Exploiting DNA damage response machinery to combat T cell exhaustion in solid tumors
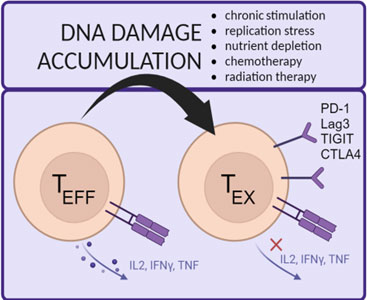 This project investigates the role of DNA damage response (DDR) in T cell function and exhaustion. In the tumor microenvironment (TME), T cells face metabolic stress and nutrient depletion, leading to increased replication-associated DNA damage. Our studies reveal that exhausted T cells accumulate DNA damage, and acute replication stress induces exhaustion, suggesting a direct link between DNA damage and T cell dysfunction. We hypothesize that enhancing DDR can prevent T cell exhaustion. To test this, we are engineering CAR T cells to overexpress key DDR proteins, assessing their function in vitro and in vivo using various preclinical cancer models.
This project investigates the role of DNA damage response (DDR) in T cell function and exhaustion. In the tumor microenvironment (TME), T cells face metabolic stress and nutrient depletion, leading to increased replication-associated DNA damage. Our studies reveal that exhausted T cells accumulate DNA damage, and acute replication stress induces exhaustion, suggesting a direct link between DNA damage and T cell dysfunction. We hypothesize that enhancing DDR can prevent T cell exhaustion. To test this, we are engineering CAR T cells to overexpress key DDR proteins, assessing their function in vitro and in vivo using various preclinical cancer models.
Population immune monitoring
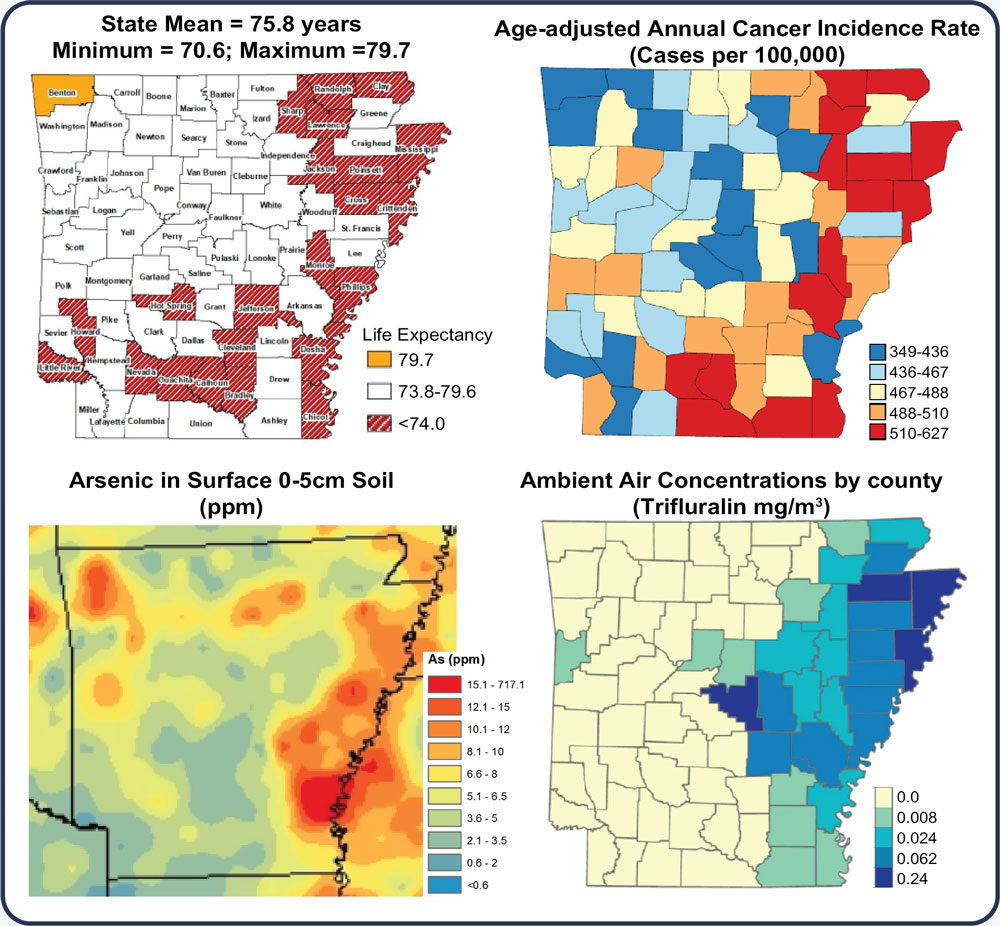
Arkansas ranks 43rd in life expectancy, in part due to high cancer incidence, low preventive health screening, poor diet, and high environmental toxicant exposures (heavy metals, pesticides). Additionally, low population mobility results in prolonged environmental exposure and accelerated epigenetic age (DNA methylation informed), which correlate to diminished immune responses. How these factors impact the response to immune-based therapies for cancer has not been fully explored. The ability to predict a therapeutic benefit and the onset of immune-related adverse events (irAE) prior to treatment would undoubtedly extend lives. Dr. Koss and collaborators seek to leverage interdisciplinary expertise in epidemiology, aging, cancer biology, immunology, and bioinformatics to build the infrastructure required to study population immune signatures associated with immune checkpoint blockade (ICB) and Chimeric Antigen Receptor (CAR) T cell therapy response.